number of protons is the same as number of electrons
it is electrically neutral unless there is one more of 1
|
atomic number
proton amount= atomic number
represented by the symbol z
also known as proton number
|
nucleon number
proton amount+neutron amount = nucleon number
represented by the symbol a
also known as mass number
|
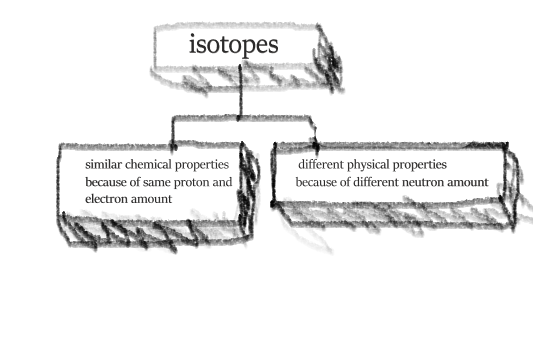
isotopes
atom of elements except it has different neutron amount
it occurs naturally
it has same number of protons and electrons but different amount of neutrons
examples include
chlorine 35(1735Cl) and chlorine 37(1737Cl)
hydrogen 2 (12H) and hydrogen 1 (11H)
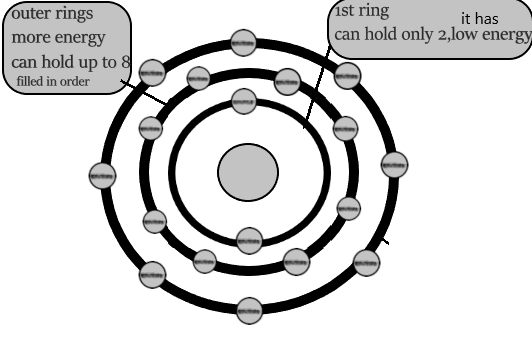
electrons move around nucleus in region known as electron shells
it corresponds to a specific energy level
it can hold only specific number of electrons
2 for the first shell and 8 for the rest of the shells
the stable electronic config of noble gas
the noble gasses are enumerated as follows
helium He (2) Neon Ne (2,8), argon Ar (2,8,8), krypton Kr (2,8,8,8), xenon Xe (2,8,8,8,8), radon Rn (2,8,8,8,8,) oganneson (im not gonna try to find the config)
noble gasses are monoatomic and exist individually,
they do not form compounds
they have full electronic shells (helium having 2 valence (duplet) and the rest having 8 (octet)
the rest of the atoms try to acheive this?
how do they?
by forming ions or molecules
i will talk about ions
they either
their outer/valence electrons
when they lose or gain they form ions
negative form cations
positive form anions

